Practitioners Must Only Use Authorized Medical Devices
A Health Canada consumer advisory about the safety of an unauthorized device should also serve as a warning to aesthetics practitioners. Be careful. Be safe.
Never purchase or use any medical device that hasn’t been approved by Health Canada.
First and foremost, it’s about patient safety. That must be our top priority as medical professionals
It’s also about liability. A practitioner risks serious legal and career repercussion in the event of a botched treatment with an unapproved medical device. An insurance company might not cover the legal costs of a lawsuit. Culpable practitioners could also be reported and lose their license.
If you are wondering if a laser, derma filler or any other medical device used in cosmetic treatments has been approved for particular treatments, do some research to make absolutely sure. Before it’s too late.
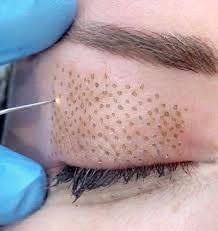
Health Canada issued the warning about the potential negative effects of a type of cosmetic device called a plasma pen.N
Health Canada is advising consumers that plasma pens (also known as ‘fibroblast’ devices), promoted for cosmetic skin treatments such as eyelid lifts, wrinkle reduction and removal of moles, skin tags, scars and spots, may pose health risks.
Plasma pens are small hand-held medical devices that focus electricity on the skin and cause a controlled burn that spreads heat throughout the targeted area.